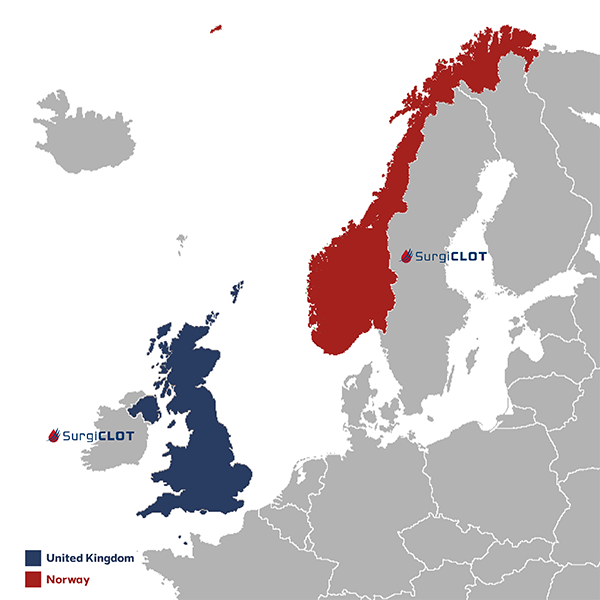
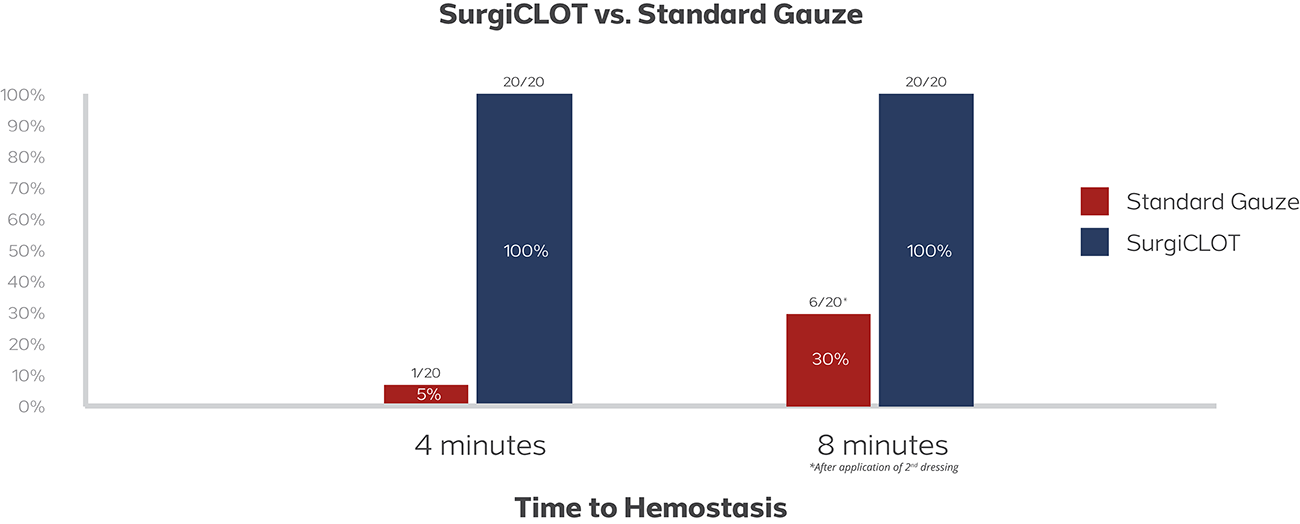
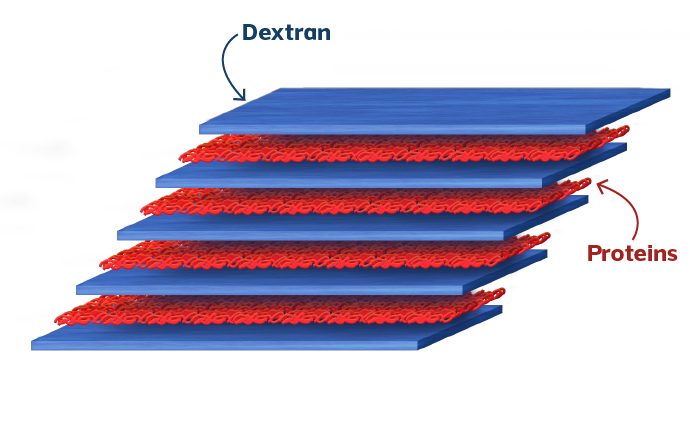
Clinical Safety and Performance Study in the United Kingdom and Norway
How safe and effective is SurgiCLOT at controlling bone bleeding?
- 30 patients enrolled
- Bone sites: 39 sites in spine and pelvis
- 90% control of bleeding at first site
- 100% control of bleeding at second site
- No significant device-related adverse events
- All surgeons reported SurgiCLOT was “as effective” or “much more effective” than other hemostatic products
- Conclusion: SurgiCLOT is safe and effective at controlling bleeding compared to other hemostatic products